Study shows how marine mammals pack muscle cells with
oxygen-holding protein
(September 26, 2015)
The ultra-stable properties of the proteins that allow deep-diving
whales to remain active while holding their breath for up to two hours could
help Rice University biochemist John Olson and his colleagues finish a 20-year
quest to create lifesaving synthetic blood for human trauma patients.
CAPTION: From
left, Rice University biochemists George Phillips, Premila Samuel and
John Olson use a
3-D visualization facility to study the structure and function of myoglobin
(red)
in the hopes of
making recombinant hemoglobin as artificial blood for use in transfusions.
Myoglobin and
hemoglobin use a molecule called heme (green) to transport oxygen.
CREDIT: Jeff
Fitlow/Rice University
In a new study featured this week in the Journal of
Biological Chemistry, Olson and colleagues George Phillips, Lucian Smith and
Premila Samuel compared the muscle protein myoglobin from humans, whales and
other deep-diving mammals. Myoglobin holds oxygen for ready use inside muscle
cells, and the study found that marine mammals have ultra-stable versions of
myoglobin that tend not to unfold. The researchers found that stability was the
key for cells to make large amounts of myoglobin, which is explains why
deep-diving mammals can load their muscle cells with far more myoglobin than
humans.
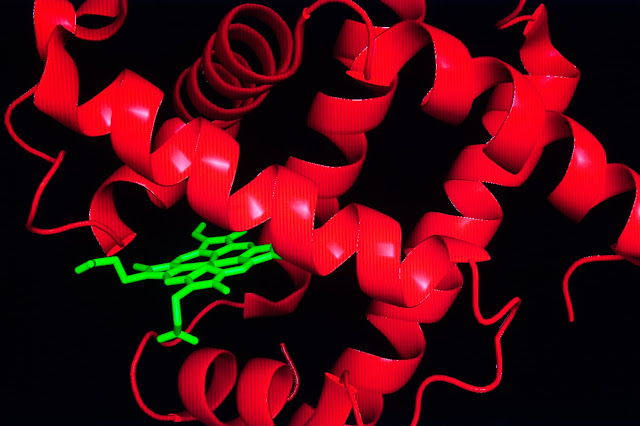
CAPTION: The shape
of myoglobin (red) includes a waterproof pocket that is used to store
heme (green), a
molecule that allows myoglobins and hemoglobins to transport oxygen.
CREDIT: Jeff
Fitlow/Rice University
“Whales and other deep-diving marine mammals can pack 10-20
times more myoglobin into their cells than humans can, and that allows them to
‘download’ oxygen directly into their skeletal muscles and stay active even
when they are holding their breath,” said Olson, Rice’s Ralph and Dorothy
Looney Professor of Biochemistry and Cell Biology. “The reason whale meat is so
dark is that it’s filled with myoglobin that is capable of holding oxygen. But
when the myoglobin is newly made, it does not yet contain heme. We found that
the stability of heme-free myoglobin is the key factor that allows cells to
produce high amounts of myoglobin.”
That’s important to Olson because he wants to create a
strain of bacteria that can generate massive quantities of another protein that’s
closely related to myoglobin. Olson has spent two decades studying hemoglobin,
a larger, more complex oxygen-carrying protein in blood. Olson’s goal is to
create synthetic blood for use in transfusions. Hospitals and trauma
specialists currently rely on donated whole blood, which is often in short
supply and has a limited storage life. A crucial part of Olson’s plan is
maximizing the amount of hemoglobin that a bacterium can express.