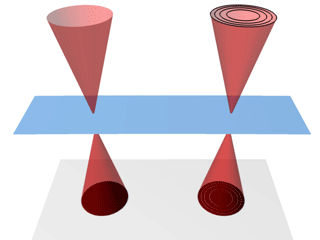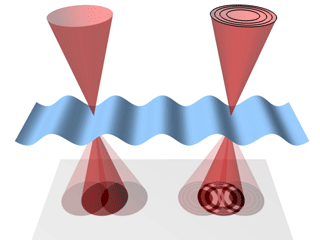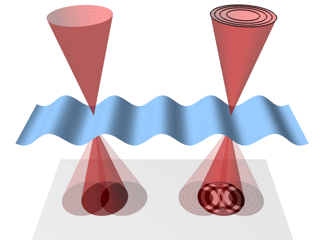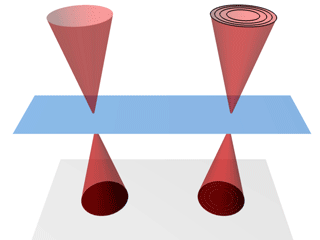
A schematic of the
nano-biosystem (top) and an electron
microscope image of quantum rods
(February 29, 2016) New
article from Maye Research Group draws on nanoscience, self-assembly
Chemists in Syracuse University’s College of Arts and
Sciences have made a transformational advance in an alternate lighting
source—one that doesn’t require a battery or a plug.
Associate Professor Mathew Maye and a team of researchers
from Syracuse, along with collaborators from Connecticut College, have recently
demonstrated high-efficient energy transfer between semiconductor quantum rods
and luciferase enzymes. Quantum rods and luciferase enzymes are nanomaterials
and biomaterials, respectively. When combined correctly, these materials
produce bioluminescence—except, instead of coming from a biomaterial, such as a
firefly enzyme, the light eminates from a nanomaterial, and is green, orange,
red, or near-infrared in color.
The findings are the subject of a recent article in ACS Nano
(American Chemical Society, 2016).
“Think of our system as a design project," Maye says.
"Our goal has been to build a nano-biosystem that's versatile enough to
teach us a lot, while allowing us to overcome significant challenges in the
field and have practical applications. The design involves materials from our
chemistry and biology labs, as well as various nanoscience and self-assembly
tools. It's a true team effort with multiple collaborations.”
Maye illustrates his point by referencing quantum rods, each
of which is four nanometers wide and 50 nanometers long. (A nanometer is 1 billionth
of a meter.) “The rods were chemically synthesized with amazing precision,” he
says. “To get the best information, we realized that we needed at least two
different types of rods, each with three synthetically tuned variations, and up
to 10 different assembly conditions.”
Having a wide range of variables has enabled Maye and his
team to learn more about the science of nano-biology energy transfer.