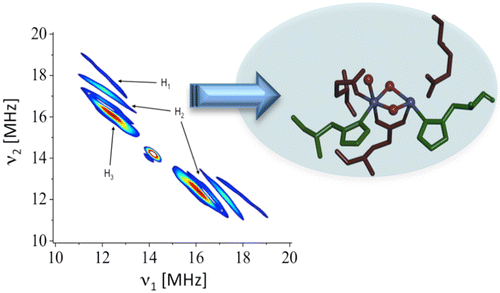
CAPTION: The new
spectroscopy method, "2D HYSCORE," is able to capture
the reactions that
split water and hydrogen peroxide in metal-containing proteins
or metallo-enzymes
in nature. CREDIT: RPI
(September 15, 2015) A
new spectroscopy method is bringing researchers at Rensselaer Polytechnic
Institute (RPI) closer to understanding – and artificially replicating – the
solar water-splitting reaction at the heart of photosynthetic energy
production. Understanding the
step-by-step mechanism of photosynthesis could lead to methods of producing
highly efficient solar energy. The
spectroscopy method, a modification of “2D HYSCORE,” is able to capture
the reactions that split water and hydrogen peroxide in metal-containing
proteins or metallo-enzymes in nature.
The researchers, led by Rensselaer professor K.V. Lakshmi,
developed the method as part of an ongoing investigation into the
photosynthetic protein, Photosystem II. Details of the research
“Two-Dimensional HYSCORE Spectroscopy of Superoxidized Manganese Catalase: A
Model for the Oxygen-Evolving Complete of Photosystem II” were published in the
Journal of Physical Chemistry.
“The solar-powered water-splitting photosynthetic protein
complex, Photosystem II, catalyzes one of the most energetically demanding
reactions in nature by using light energy to split water to dioxygen,” said
Lakshmi, associate professor of chemistry and chemical biology, and scientific
director at the Baruch ’60 Center for Biochemical Solar Energy Research at
Rensselaer. ”However, the details of the water-splitting reaction have remained
elusive due to the inability of conventional methods to probe the active site
of metal-containing proteins, like Photosystem II.”
Photosystem II, found in plants and cyanobacteria, uses
photons of light to break apart molecules of water, extracting electrons and
protons to fuel the photosynthetic conversion of light and water into chemical
energy that is used to power the planet. This reaction – solar oxidation of
water – takes place in a cluster of oxygen, manganese, and calcium ions called
the “oxygen-evolving complex.” The oxygen-evolving complex uses four photons of
light to split two molecules of water in five distinct steps known as
“S-states.” Each intermediate S-state, numbered from S-0 to S-4, is measured in
trillionths of a second, and the final three S-states (S-2, S-3, and S-4)are
highly unstable, making it difficult to determine the exact mechanism by which
they occur.